Table of Contents
- General Questions
- Setting Up An Account
- Close Concerns Knowledgebase Home Page FAQ
-
Closer Look Mobile App
- How do I turn on or off the push notifications?
- How do I personalize my push notifications?
- What platforms is the app available on?
- How do I access the search engine?
- How do I save reports into my customized library? How do I remove reports from my library?
- Can I save reports for offline use?
- How can I submit feedback regarding the app?
- Useful Search Tips, Tricks, and FAQ
- Notation and Abbreviation Conventions
General Questions
What is the Close Concerns Knowledgebase?
The Close Concerns Knowledgebase is a website where you can find the most recent Closer Look reports that we have published as well as search our historical archive. Closer Look is a periodic news service focused on new developments in diabetes and obesity that you receive via email several times each week from Kelly Close.
For a more in-depth introduction to the Knowledgebase, please see our introduction video here.
Setting Up An Account
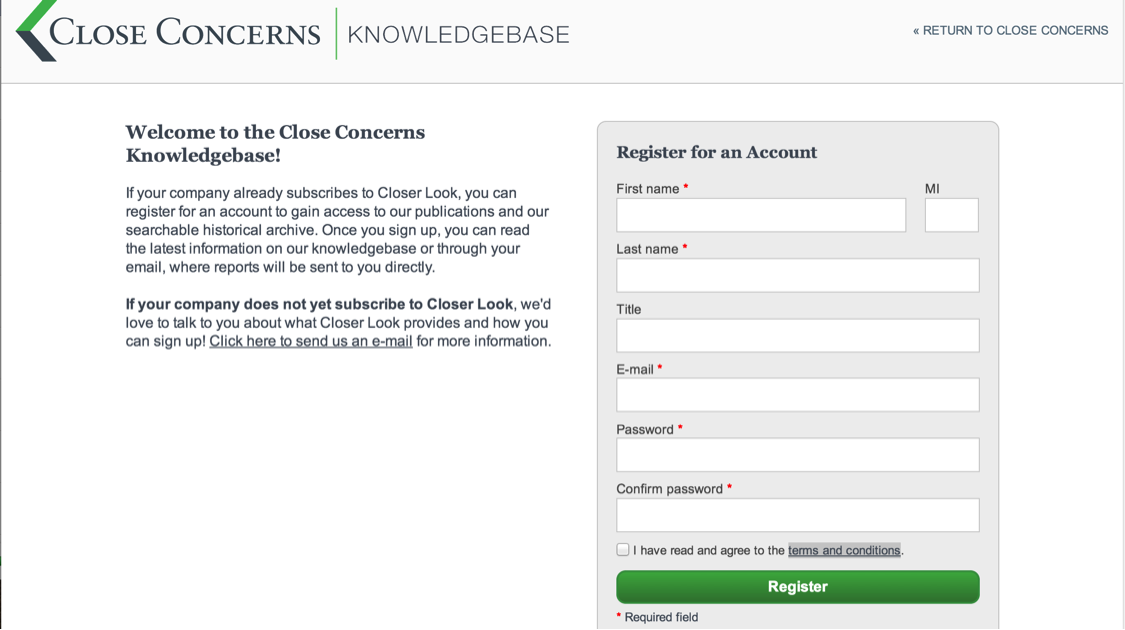
How can I change the settings in my account?
To change the settings on your account, go to "My Account" in the top, right hand corner (in the side slider menu on the app).
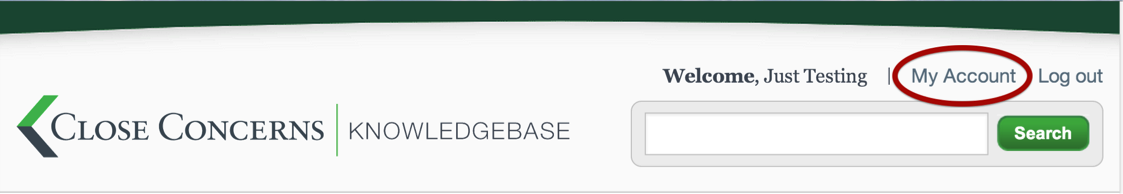
Once on the “My Account” page, you may edit your name, title, password, and email address.
Close Concerns Knowledgebase Home Page FAQ
How do I navigate to the home page?
You can click on the Close Concerns Knowledgebase logo at the top of any page to return to the home page.
What articles show up on the home page?
The most recent reports we have published will show up on the home page organized by category (drugs, technology, conferences, obesity, etc.). Reports will typically be published to the homepage somewhat earlier than you receive the email compilation of recent reports via e-mail from Kelly.
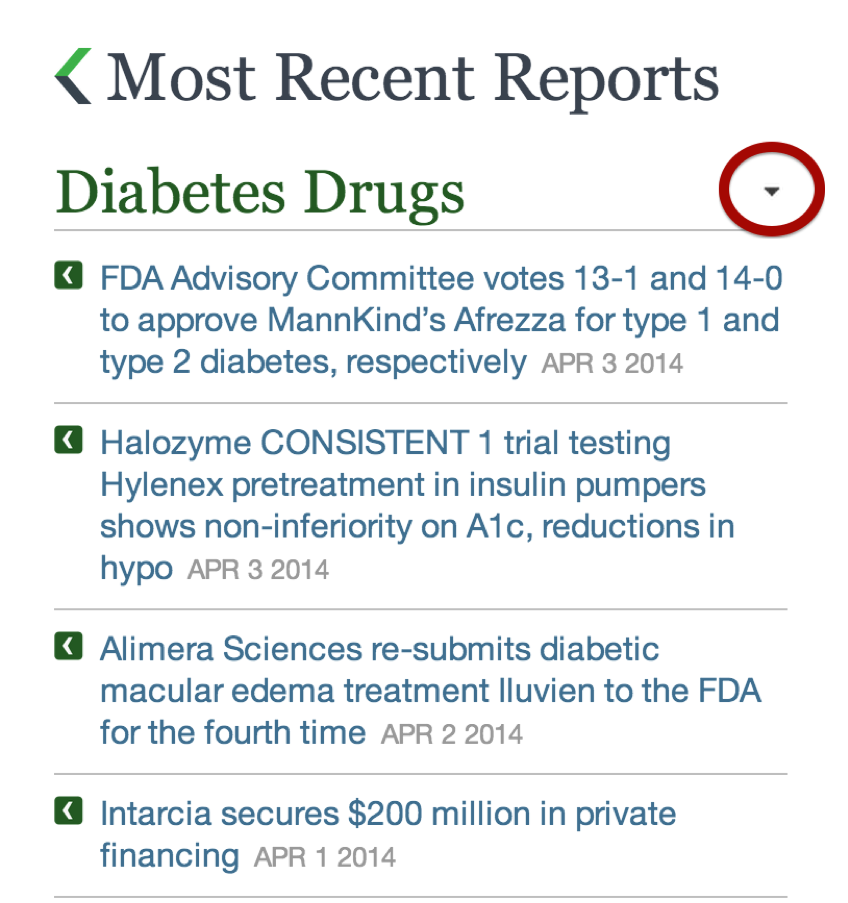
How do I collapse or expand each category?
You can collapse or expand each category by clicking the arrow to the right of the category name.
Closer Look Mobile App
How do I turn on or off the push notifications?
Go to your iOS device’s settings and find notifications. Within the notifications section, find the Closer Look app and tap "Allow Notifications" to turn on or off your notifications.
Please note that we often publish our reports during late hours on Pacific Standard Time (PST), which may send push notifications at inconvenient times for you. If you would like to avoid this disturbance, we recommend setting your device to "Do Not Disturb" during certain time periods or turning the sound off within your device's notification settings for the Closer Look app.
How do I personalize my push notifications?
To personalize your push notifications, go to your "My Account" page and under "Personalize Your Notifications," please only check the boxes of the coverage area categories (Diabetes Drugs, Diabetes Technology, Conferences, Obesity, Big Picture, Also) from which you would like to receive notifications.
What platforms is the app available on?
Currently, the Closer Look app is only available on the iOS platform. If you have thoughts on other platforms, we would love to hear them through the app’s “Submit Feedback” section.
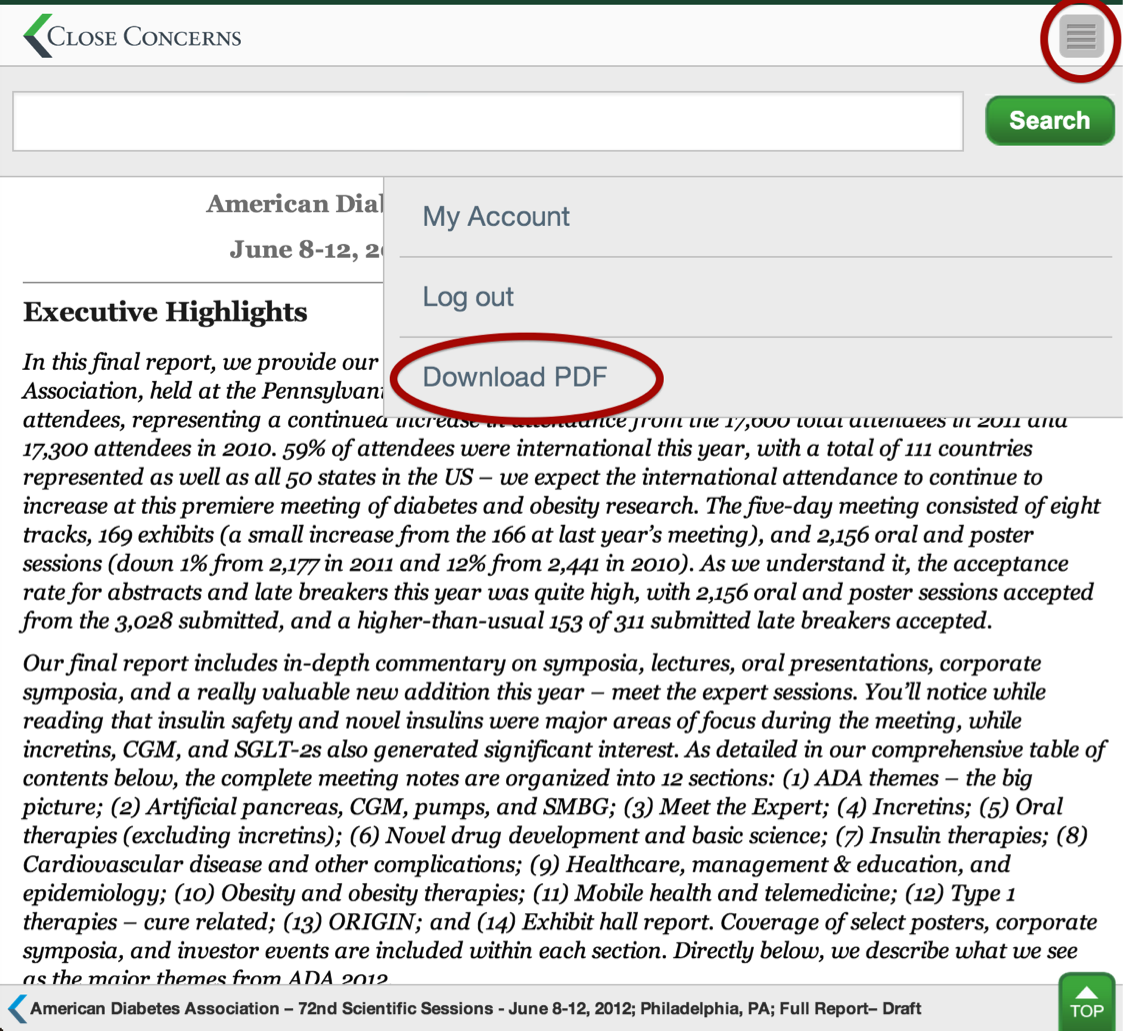
How do I access the search engine?
The search bar can be found by tapping the side slider menu at the top, right hand corner. From here, you can enter any query to search the Closer Look archives.
How do I save reports into my customized library? How do I remove reports from my library?
You should see an empty star next to each report on the homepage, in your search results, as well as within each report itself. By tapping this star, the star will appear filled in and your report will be saved into the section “My Library” (in the side slider menu). To remove reports from your library, just tap the star again to return it back to an empty star.
This feature is also available on the desktop version of the Close Concerns Knowledgebase.
Can I save reports for offline use?
At this time, the app does not allow for saving reports for offline use. If interested, we would love to hear your feedback through the app’s “Submit Feedback” section.
How can I submit feedback regarding the app?
You will find the “Submit Feedback” section in your side slider menu (click on the menu at the top, right-hand corner) and enter in your name, e-mail address, and feedback. We are always trying to improve your experience so any input is greatly appreciated.
Useful Search Tips, Tricks, and FAQ
What is the scope of the content in the Knowledgebase?
It may help to understand what is and isn't contained in our archives to guide your searches. The Knowledgebase contains our coverage of diabetes and obesity-related industry updates from 2009 to present; it doesn’t contain detailed coverage of other disease areas. Each of our reports is usually written about a specific piece of recent news, so you’ll generally be more successful if you know which piece of information you’re looking for, or which report you’re looking for.
We publish six main types of reports:
- Conference Reports
- Individual News Items (a report with a single headline on a specific update, such as a product launch, company merger, topline data release, or news of regulatory progress)
- Quarterly Earnings Reports (quarterly financial and pipeline reviews for the public companies we regularly report on)
- FDA Advisory Committee Meeting reports
- Interviews with key opinion leaders
- Summaries of recent scientific literature articles (sometimes called “literature reviews” in our archives)
How do I access the search engine?
The search bar can be found at the top, right hand corner of the Close Concerns Knowledgebase home page. From here, you can enter any query to search the Closer Look archives.

How can I sort search results?
Above the list of search results, there are two drop down menus that can change the way results are ordered. The default option is to sort by Relevancy, but it is also possible to sort by Date and Title.
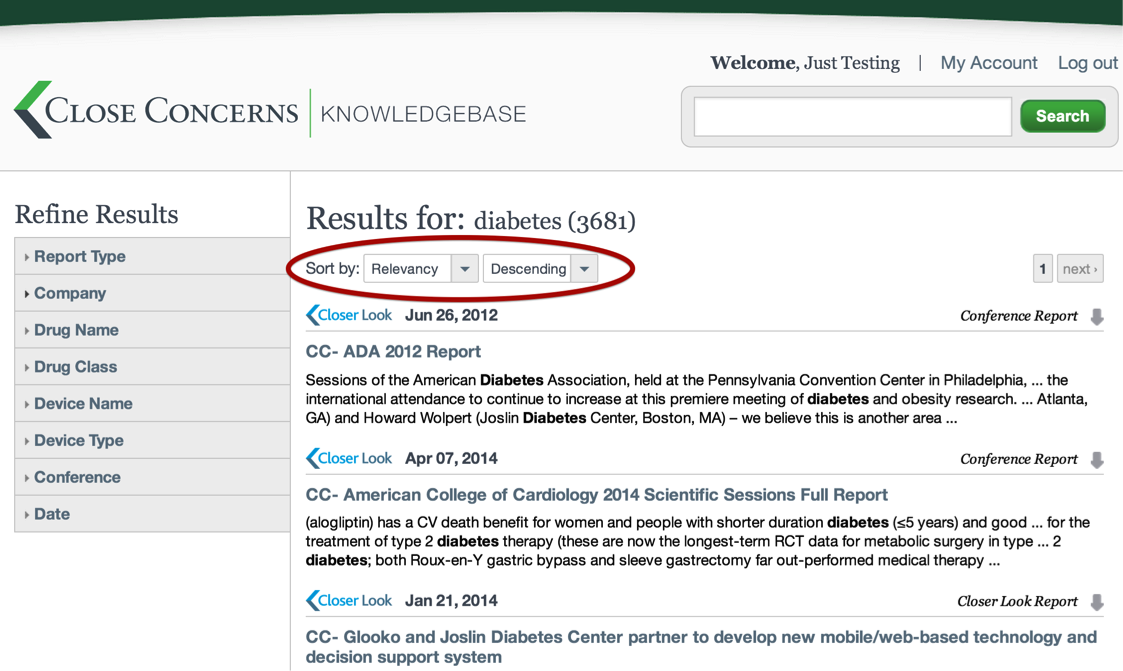
That’s a lot of articles! How can I refine the search results?
Using quotation marks around exact phrases: For example, if I search for ORIGIN trial, I’ll get results that contain the word “origin” or the word “trial” anywhere in the report. Because these two search terms are so general, the query is not specific enough to find what I am looking for. Putting this phrase in quotes returns much more specific results. Be careful not to overuse quotes, though, as the more specific your query is, the more limited your results will be.
Using Boolean operators and other commands:
- AND: Searching glucagon AND Lilly will exclusively return results that have both of these terms in the document.
- OR: Searching glucagon OR Lilly will return results that have either of the two terms.
- NOT: Searching glucagon NOT Lilly will return results that contain glucagon but not Lilly.
- Using the + sign in front of a term (e.g., +pump) will require all search results to contain that term.
- Using the – sign in front of a term (e.g., -pump) will exclude any results with that term.
- The wildcard symbol, *, can be placed at the end or in the middle of any term. For example searching for monito* will pull up results containing “monitor” or “monitoring"; submi* will pull up results containing "submitted" or "submits" or "submission"; and F*rxiga will pull up results containing either "Forxiga" or "Farxiga."
- If you are unsure how to spell something, you can do a fuzzy search: To do a fuzzy search use the tilde, "~", symbol at the end of a single word term. For example to search for a term similar in spelling to "roam" use the fuzzy search roam~ (this will find results like “foam” and “roams”).
- If you want to search for two terms that may be near each other but not an exact phrase, you can perform a proximity search. To do a proximity search use the tilde, "~", symbol at the end of a phrase in quotations. For example the search “CGM penetration”~10 will find results with "CGM" and "penetration" within 10 words of each other.
- To boost the importance of one term over others, you can use a caret with a boost factor (number) at the end of the search. For example, searching for Tandem^3 insulin pump will boost the relevance of the word Tandem three times that of insulin or pump.
Using filters: On the left side of the screen (or the top of the screen if you are using a tablet or mobile device), you will see a series of search filters. You can refine results by Type of Report (for example conference reports, quarterly earnings reports, interviews etc.), Company, Drug Name, Drug Class, Device Name, Device Type, and Conference name.
Once a filter is selected, only documents fitting that filter will appear in the search results.
If you are having trouble finding a report, you may find it helpful to consult the section of this page titled “Notation and Abbreviation Conventions” for details on how organization names may be abbreviated in report titles, which may help you find alternative search terms to try.
Notation and Abbreviation Conventions
Title conventions
- Daily highlights from conference reports will have the word "Day" in the title
- Full conference reports, which provide our full, in-depth coverage of a conference and are typically published after the conference is over, will have the phrase "Full Report" in the title
- Quarterly financial update reports are titled "Company name #QYY" (e.g., "Dexcom 4Q12").
- When companies' fiscal quarters are offset from calendar quarters, we title documents using the fiscal quarter (e.g., "Array F2Q13" for Array's calendar 4Q12 report).
- Company abbreviations that may be used in titles (this list is not exhaustive, so please let us know if we’ve left out an abbreviation that you’re having a hard time decoding!)
- AstraZeneca: AZ
- Becton Dickinson: BD
- Boehringer-Ingelheim: BI
- Bristol Myers Squibb: BMS
- GlaxoSmithKline: GSK
- Johnson & Johnson and Janssen: we refer to both as J&J
- Novo Nordisk: NN
Typically, we will title a conference or meeting report using the name of the organization hosting the conference followed by the year (e.g., "ADA 2013"). Common conference abbreviations include:
- American Association of Clinical Endocrinologists: AACE
- American Association of Diabetes Educators: AADE
- American College of Cardiology: ACC
- American College of Physicians: ACP
- American Diabetes Association: ADA
- American Heart Association: AHA
- American Society Clinical Pharmacology Therapeutics: ASCPT
- Advanced Technologies and Treatments for Diabetes: ATTD
- Consumer Electronics Show: CES
- Committee for Medicinal Products for Human Use: CHMP
- Centers for Medicare and Medicaid Services: CMS
- Controversies to Consensus in Diabetes, Obesity, and Hypertension: CODHy
- Children With Diabetes: CWD
- Diabetes Technology Meeting: DTM
- European Association for the Study of Diabetes: EASD
- Excellence in Diabetes: EiD
- European Congress on Obesity: ECO
- European Medicines Association: EMA
- The Endocrine Society: ENDO
- Food and Drug Administration: FDA
- International Diabetes Federation: IDF
- International Society for Pediatric and Adolescent Diabetes: abbreviated as ISPAD
- The JP Morgan Healthcare Conference: JPM or JP Morgan
- Metabolic Disease and Drug Development: MDDD
- Partnership for a Healthier America: PHA
- Society of Critical Care Medicine: SCCM
- The Obesity Society: TOS (the TOS and ASMBS annual meetings joined forces starting in 2013 in a conference known as "Obesity Week")
Close Concerns Knowledge Base Index
Below you’ll find an index of frequently used acronyms, terms, and phrases in Closer Look. We hope this proves to be a valuable resource in navigating the ever-expanding field of diabetes therapy and technology. We imagine that this resource will similarly grow with time. If you have suggestions for how to improve this page, or terms you think should be added – let us know!
Terms are listed in alphabetical order and broadly divided between diabetes therapy, diabetes technology, and obesity. We recommend using the “control+F” search function to find specific terms.
Diabetes Therapy
A1c: Hemoglobin A1c, glycated hemoglobin. Measures glucose bound to hemoglobin in red blood cells, and is used to approximate average blood glucose levels over three month periods.
ADA: American Diabetes Association.
AZ: AstraZeneca.
CHMP: Committee for Medicinal Products for Human Use. Part of the European Medicines Agency, it is responsible for reviewing human medicines in the European Union.
CI: Confidence Interval.
CKD: Chronic Kidney Disease. Condition that involves progressive loss of kidney function.
CRL: Complete Response Letter. Issued after FDA has completed review of a New Drug Application (NDA) and indicates that it cannot approve the drug without additional data or information.
CV: Cardiovascular.
CVD: Cardiovascular Disease.
CVOT: Cardiovascular Outcomes Trial. Long-term trials that must be conducted for antidiabetic drugs following a 2008 FDA mandate to determine potential cardiovascular risk.
DCCT: Diabetes Control and Complications Trial. Landmark trial that demonstrated that type 1 patients with intensive glycemic control had fewer diabetes-related complications compared to those using conventional treatment. Microvascular complications including retinopathy, nephropathy, and neuropathy were all significantly reduced in the intensive treatment group. As a result of DCCT/EDIC, early and intensive glucose control is the standard of care for people with both type 1 and type 2 diabetes.
DKA: Diabetic Ketoacidosis. Serious complication marked by high levels of acidic blood ketones.
DME: Diabetic Macular Edema
DKD: Diabetic Kidney Disease. Diabetes-related complication that involves progressive loss of kidney function. Also known as diabetic nephropathy.
EASD: European Association for the Study of Diabetes.
DPP-4 inhibitor: Dipeptidyl peptidase-4 inhibitor. Diabetes therapy that serves to increase insulin secretion and decrease glucagon levels in order to reduce blood glucose levels.
EDIC: Epidemiology of Diabetes Interventions and Complications Study. Ongoing follow-up to the DCCT that has shown long-term benefits of early and intensive blood glucose control on the future development of diabetes-related complications. As a result of DCCT/EDIC, early and intensive glucose control is the standard of care for people with both type 1 and type 2 diabetes.
eGFR: Estimated Glomerular Filtration Rate. Measured used to determine kidney function; lower levels indicate declining kidney function.
EMA: European Medicines Agency.
FDA: Food and Drug Administration.
GLP-1 RA: Glucagon-like Peptide-1 Receptor Agonist. Type 2 diabetes therapy that stimulates insulin secretion and inhibits glucagon secretion, thereby lowering blood glucose levels.
HCP: Healthcare Provider.
HF: Heart Failure.
HFpEF: Heart Failure with Preserved Ejection Fraction.
HFrEF: Heart Failure with Reduced Ejection Fraction.
HHF: Hospitalization for Heart Failure.
HR: Hazard Ratio.
IND: Investigational New Drug application. FDA application for which manufacturers must have accepted in order to test a potential drug in human clinical trials.
hsCRP: High Sensitivity C-Reactive Protein. Quantifying levels of this protein is often used as a measure of inflammation.
JAMA: Journal of the American Medical Association.
MACE: Major Adverse Cardiovascular Events. Composite endpoint frequently used in cardiovascular outcomes trials (CVOTs). Three-point MACE is defined as the composite of non-fatal stroke, non-fatal myocardial infarctions, and cardiovascular death.
NAFLD: Nonalcoholic Fatty Liver Disease. Characterized by fat buildup in the liver due to causes other than alcohol use. NAFLD can progress to the more serious nonalcoholic steatohepatitis (NASH).
NASH: Nonalcoholic Steatohepatitis. Condition characterized by liver inflammation and damage caused by buildup of fat in the liver. NASH is highly comorbid with diabetes and obesity. NASH is the progressive and more serious form of nonalcoholic fatty liver disease (NAFLD).
NDA: New Drug Application. Comprehensive document that a sponsor must submit to FDA to get approval required to market a new drug in the US. FDA has 60 days to reach a decision on an NDA.
PDUFA date: Prescription Drug User Fee Act date. FDA target decision date for review of an NDA (New Drug Application).
PK/PD: Pharmacokinetic/Pharmacodynamic.
RRR: Relative Risk Reduction. Relative decrease in the risk of an adverse event in a treatment group vs. control group.
SGLT inhibitor: Sodium-glucose co-transporter inhibitor. Small molecule diabetes therapy that blocks reabsorption of glucose by the kidney.
YOY: Year Over Year.
Diabetes Technology
AID: Automated Insulin Delivery. Use continuous glucose monitors (CGMs) and algorithms to automatically adjust insulin delivery via pump.
ADS: Automated Decision Support. Rule-based systems that can automatically provide solutions to repetitive management problems. A forward looking glucose forecast up to 12 hours ahead used for a small group of non-insulin dependent type 2 users with One Drop.
BGM: Blood Glucose Monitor.
CDER: Center for Drug Evaluation and Research.
CDRH: Center for Devices and Radiological Health.
CGM: Continuous Glucose Monitor. System that tracks glucose levels by taking glucose measurements at regular intervals 24/7.
CMS: Centers for Medicare and Medicaid Services.
DIY: Do-it-Yourself.
DME: Durable Medical Equipment.
DPP: Diabetes Prevention Programs.
DTM: Diabetes Technology Meeting.
ECG: Electrocardiogram.
EHR: Electronic Health Records.
EMR: Electronic Medical Record.
FFL: Friends for Life. Annual diabetes conference in Orlando, FL to learn about the most current information in diabetes care. Organized by the Children with Diabetes, Inc.
HCL: Hybrid Closed Loop. A pump system that is intended for continuous delivery of basal insulin.
iCGM: Integrated Continuous Glucose Monitor. A type of CGM with “special controls” to ensure safety/effectiveness and interoperability.
JDRF: Juvenile Diabetes Research Foundation.
MDI: Multiple Daily Injections.
MGH: Massachusetts General Hospital.
MIDS: Mobile Insulin Dosing System. Interactive application that helps people with type 2 diabetes titrate their long-acting insulin.
NIDDK: National Institute of Diabetes and Digestive and Kidney Diseases.
NN: Novo Nordisk.
OUS: Outside the United States.
PCP: Primary Care Physicians.
PDM: Personal Diabetes Manager. An Omnipod device which is the control center for checking blood sugars, giving insulin, and has a food database and logbook.
PMA: Premarket Approval.
RCT: Randomized Control Trials.
SMBG: Self Monitoring of Blood Glucose.
TIR: Time in Range (70 mg/dL-180 mg/dL)
TAR: Time above Range (>180 mg/dL)
TBR: Time below Range (<70 mg/dL)